Over 25 years of
About Us
Founded in 1986, VEI was born out of unbridled passion to meet the unmet and emerging needs faced by medical manufacturers. At a time when manufacturing processes were advancing at an ever-increasing rate and FDA regulation continued to expand it was clear there was a need to protect against the inevitable product mix.
Since our infancy, we've grown with FDA regulations by systematically designing solutions with Title 21 CFR Part 11 compliance and validation in mind. Throughout our history, VEI has expanded from providing solutions based purely on product mix avoidance to a comprehensive suite of quality centered solutions. Our offerings consistently reduce cost & mitigate risk, creating a powerful package making justification common sense. In fact, our clients repeatedly tell us that our technology has successfully prevented many CAPAs and recalls.
At VEI we're passionate about living with purpose and wholeheartedly believe that by protecting our clients and their patients we're making a meaningful difference. In an unforgiving industry where there's no room for mistakes we've built our business on dedication to our customer and their success. This is what we're all about, we're customer centered, market driven innovators who develop robust solutions where others only see problems. We're going to continue to question the status-quo, develop solutions for the needs of the industry & play a critical role in advancing medical manufacturing for the better.
Common Clients Include
global presence
Industries served
Implantable Devices
Implantable device manufacturers face many challenges in the current landscape. As Current Good Manufacturing Practices (CGMP) are continually refined and evolve each change offers new benefits but also presents unique challenges. Additionally, operator subjectivity and the possibility for human error have continually presented problems for manufacturers.
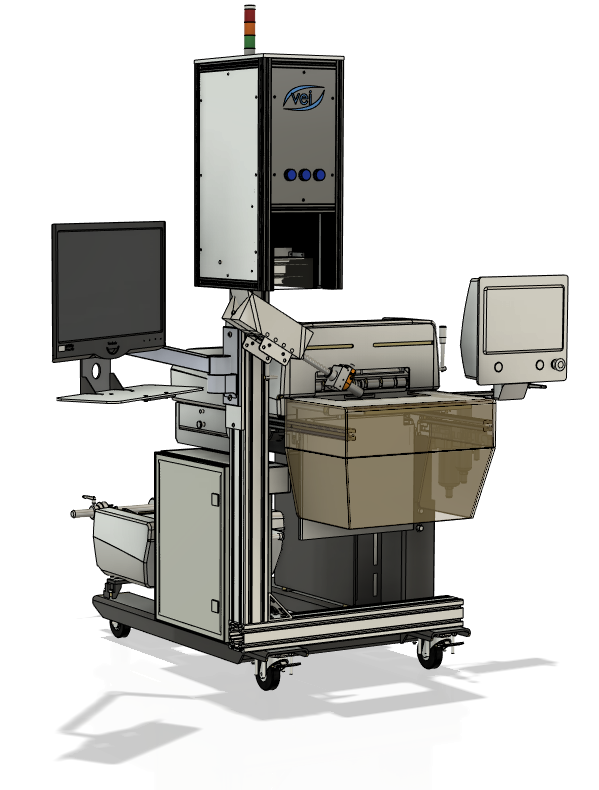
Throughout our history VEI has developed solution based products to meet the industry’s current needs. Product mix, quality defects, software discrepancies, and mislabeling are a few of the problems VEI’s product lineup addresses. Our solutions employ a variety of technology to successfully mitigate risk while further reducing cost. Common medical device sectors include orthopedics, ortho recon, maxillofacial, spine, & dental.
Cardiovascular Implants
Comparable to much of the medical manufacturing community the cardiovascular industry faces increasing regulation and compliance measures both locally and abroad. Furthermore, in an industry where one mistake is one too many the demands placed on manufacturers continue to grow. Challenges are in part due to increasing pressure to further miniaturize devices, material reduction, and implementation of improved manufacturing methods. With these challenges quality checks can become increasingly difficult and as such the absence of all errors is simply not realistic. VEI offers both custom and off the shelf packages designed to meet these needs.
Biologics
The biologics industry faces complex manufacturing processes in part due to fermentation, aseptic processing, storage & testing requirements. Hence, it’s understood that biologics are more expensive than simple chemically fabricated drugs. Additionally, due to biologics intended use often dictating FDA classification drugs can fall into categories of both a medical device and biologics. This results in extensive quality control measures focusing heavily on the drug’s assays and the like. Nevertheless, few systems adequately protect against drug, label, or other product mix situations. At VEI we provide solutions to meet this need and mitigate risk.
Clinical Trials
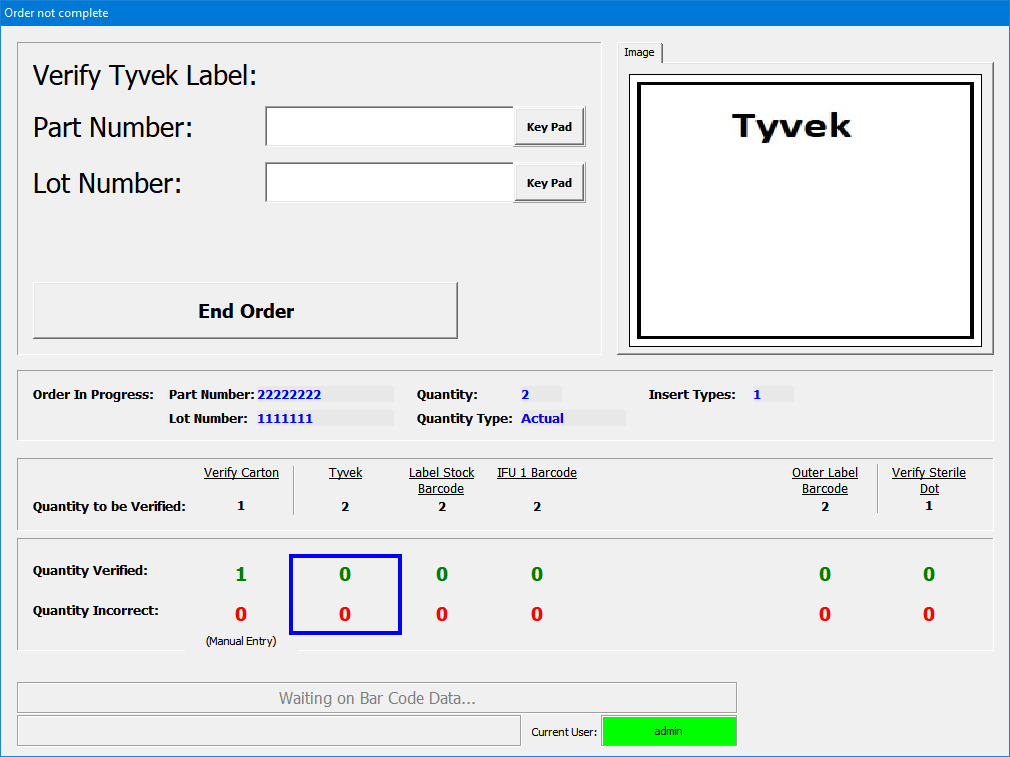
VEI offers several solutions which can assist with FDA requirements concerning the control of materials, labeling, reporting, and process controls.
Pharma
Offering a variety of quality based solutions for data logging, process monitoring, and including printer label verification, pill integrity, quantity, color, reporting & ERP integration.
Contact Us
Headquarters
Vision Engineering, Inc.
299 3rd Avenue
Sherman, MS 38869
Phone: 662.842.8400
Fax: 662.680.4700
Midwest Office Address
189 E Bell Drive
Warsaw, IN 46582
Brian Boatner
brianb@visionengineeringinc.com
Mobile: 662.401.8787
Office: 662.842.8400